Life as an entropic conspiracy
The right question to ask
“What is the meaning of life?”
This question has become quite a cliché, with an equally cliché answer of:
“It’s whatever you make it out to be!”
that has become the norm.
I believe the reason why such a cop-out answer is the norm is because deep down, we all understand that life has no intrinsic objective meaning1. In fact, given that the prerequisite for the concept of “meaning” to exist in the first place is the existence of life itself, this question seems rather like a trick question.
1 Sans whatever religious/spiritual beliefs that one might have.
2 e.g., the aforementioned religious/spiritual beliefs.
Similar to how a system cannot demonstrate its own consistency in Gödel’s second incompleteness theorem, perhaps one must require a framework that is external to life itself to apply “meaning” for life’s existence, before life’s existence2.
Nevertheless, by replacing the quite human term of “meaning” with a more mechanistic term like “purpose”, we might be able to answer more reasonably the resulting question of:
“What is the purpose of life?”3
3 The term “purpose” might still be quite “agentic”, in the sense that it might mislead one into thinking that life has some agency over its own evolution based on some sense of purpose that it has. I assure that this is not what this post is about.
A Physicist’s perspective — the conspiracy
The study of life is typically assumed to fall under the domain of Biology. However, physicists have also been attempting to uncover a physical principle that leads to life’s emergence and its behaviors (Prigogine and Nicolis 1971). A hint lies in the stark difference between life and inanimate matter: life’s ability to efficiently absorb or consume useful energy and dissipating it as heat, resulting in increased entropy production.
While this have been pointed out by Erwin Schrödinger in his seminar book What is life? back in the 1940s, it has undergone rigorous thermodynamic treatment in recent years, thanks to advances in the physics of non-equilibrium thermodynamics (Collin et al. 2005; K. Michaelian 2011).
In short, in the presence of an external non-equilibrium energy source (like the Sun), systems adapt and self-organize themselves to better absorb energy and dissipate heat or entropy. This process has come to be known as dissipation-driven adaptation (England 2013; Perunov, Marsland, and England 2016).
Life is hypothesized to be such a dissipative system that serves to increase entropy production.
From the birth of planetoids in the dusts of protoplanetary disks to the formation of vortices we see in chaotic turbulent flows such as hurricanes. Stable structures with low internal entropy arise out of such chaotic interactions in order to more efficiently dissipate entropy.
Life might be one of such order out of chaos, a long-running, universe-scale conspiracy to increase the entropy of the cosmos.
Self-organization — order out of chaos
Self-organization of complex structures in the presence of an external energy source have been demonstrated both experimentally and computationally. In a computational simulation involving 20 particles in a simplified chemical space, complex bonding patterns emerged under resonance with a periodic drive, increasing the rate of work absorption and heat dissipation (Kachman, Owen, and England 2017).
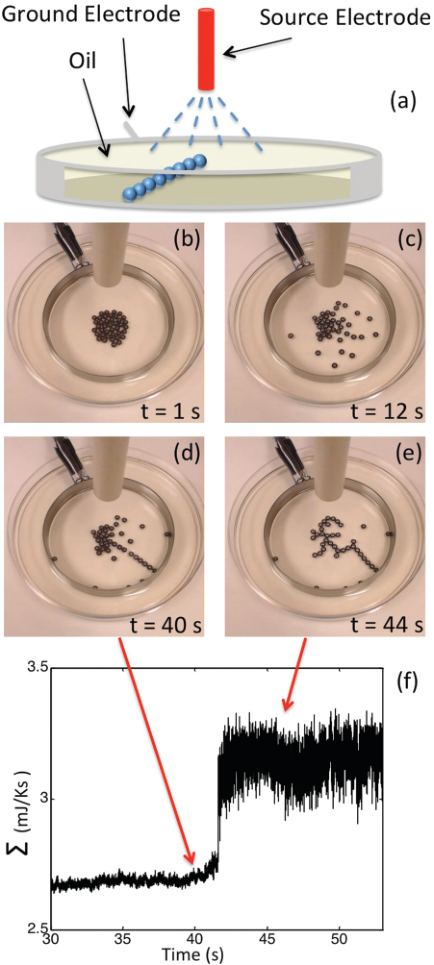
Setup and result of self-organizing conducting beads.
Experimentally, conducting beads 4mm in diameter immersed in oil in a petri dish were shown to self-organize to a complex, tree-like structure when subjected to a high voltage. This occurred at a critical point and was accompanied by a spike in entropy dissipation (Kondepudi, Kay, and Dixon 2015). Silver nanorods were also observed to self-organized in the presence of a doughnut-shaped laser source, with resulting structures depending on the wavelengths and polarization of the laser source (Ito et al. 2013).
Likewise, by considering UVC light of 230nm to 270nm from the Sun as the non-equilibrium energy source (a range of wavelengths that were most intense in the early Earth), it was demonstrated that RNA polymers could proliferate via photochemical reactions, and that DNA polymers would denature from absorption and dissipation at these UVC wavelengths. These are crucial steps for self-replication without enzymes (Karo Michaelian 2017; Karo Michaelian and Padilla 2019).
Homochirality in DNA and RNA was also shown to be achieved via photochemical reactions with UVC wavelengths light (Karo Michaelian 2018). Correlations also exist between the maximum absorbance wavelengths of early life’s nucleobases and amino acids, and UVC wavelengths (K. Michaelian and Simeonov 2015).
These findings suggest that in the RNA world during early Earth era, RNA or pre-RNA polymers may have self-organized or adapted to efficeintly absorb and dissipate UVC solar spectrum, a prime example of dissipative adaptation in the emergence of life.
Self-replication — the more the merrier
Clearly, the more dissipators there are in a system, the greater the overall entropic dissipation. It’s therefore unsurprising that complex systems can self-replicate to increase entropy dissipation.
In fact, even the act of self-replication itself can serves to dissipate heat, e.g., it was shown that E. coli bacteria dissipate at least 5 times as much heat during replication compared to individual heat dissipation (England 2013).
The process of DNA replication (copolymerisation) also dissipates entropy depending on the information entropy of the DNA polymer. Information creation decreases the Shannon entropy of the polymer and generates mutual information with the original. In the process, entropy is dissipated to the environment as quantified by the affinity per copied nucleotide (Andrieux and Gaspard 2008).
In general, various dissipative systems exhibit self-replication behaviors. For example, computer simulations of colloidal clusters show that the exponential growth rates of self-replication varies with the energy landscape of the system (Zeravcic and Brenner 2014).
Three-dimensional vortices also self-replicates in rotating shear flows, as demonstrated in a numerical study (Marcus et al. 2013). This was also proposed to be how planetoids form in protoplanetary disks (Barranco and Marcus 2005), whereby the larger system can replicate smaller dissipative systems in it, or eventually split into a copy or more of itself, i.e., disk fragmentation that forms binary star systems (Offner et al. 2010).
Given these observations, one might hypothesize that early organic compounds evolved the ability to self-replicate as a means to ensure the constant presence of entropy dissipating structures, driving the emergence and evolution of life.
Adaptation — resonance with the environment
If life indeed self-organizes to more efficiently absorb external energy sources and dissipate heat, we would expect it to adapt or change its structures in response to changes in those energy sources over time.
Correlations between solar spectrum and the emergence of organic compounds and cells in the five different periods. As Earth’s conditions changes, more complex organic compounds evolved to suit these conditions. The general trends are a shift from UVC absorbing to visible spectrum absorbing compounds, and a shift to oxygen based respiration.
This seems to be evident throughout life’s history, where its evolution has underwent avalanche dynamics with quick and sudden changes in complexity rather than smooth and gradual evolution (Paczuski, Maslov, and Bak 1996). Some even modeled these sudden transitions of evolution as a Big Bang model with “evolutionary inflation” akin to the cosmological inflation model (Koonin 2007). Simulated artificial life have also demonstrated how the fitness or how the population adapts to the environment, can undergo multiple discontinuous jumps (Adami 1995).
Correlating Earth’s historical environmental changes (like solar spectrum, temperature, and atmospheric compositions) with organic compounds’ emergence and evolution shows that life has consistently adapted to better survive and absorb energy according to the prevailing condition in that period (K. Michaelian and Simeonov 2015).
An interesting example is the case of adenosine triphosphate (ATP) synthase, an enzyme that lies along the cell membrane of a cell that breaks down ATP to adenosine diphosphate (ADP) and a phosphate. This breakage releases energy that powers the cell.
Using a kinetic model of the ATP synthase process reveals that both its entropy production and the Shannon entropy of the transitional states, are maximized at a catalytic dwell angle4 consistent with the actual ATP synthase’s angle, indicating that the ATP synthase evolved to optimize entropy production (Dewar, Juretić, and Županović 2006).
4 The ATP synthase rotates in angles of 120^{\circ} for each ATP to ADP + P_i reaction. However, at the moment of breakage of ATP bonds to ADP + P_i, the synthase will pause for a short amount of time of around 2ms (Ueno et al. 2005). The angle at which this pause happens is known as the catalytic dwell.
5 One should keep in mind that none of these results were shown for complex life such as us humans, and one should not have the illusion that this hints at some sort of “Lamarckism”.
These findings suggest that the process of self-organization to an external energy source is an ongoing, dynamic phenomenon that continues in response to changing conditions.5
The purpose of life
So why does the universe favors orders such as life, rather than simply maintaining the disorder that we might expect?
As discussed, it turns out that these eddies of order and low entropy pockets of the universe such as life, are but a small price for the universe to pay to optimize the production of entropy in the long run.
In other words, contrary to what one might expect, the presence of stable complex structures such as life produces more entropy in the system than if these structures were absent.
So it seems that the conspiracy of life is an entropic one.
This long-running conspiracy is but an attempt to speed up and maximize the production of entropy and disorder. The universe has set aside a small part of itself for us, masquerading as an exception to the Second Law of thermodynamics, but is in fact a testament of its power.
This is the purpose of life, the unwitting servitude as an agent of entropy.